The success of his method of making steel made Bessemer a prolific businessman, reaching an 80% share of the railway steel market in the late 19th century. Credit: Wikimedia Commons.
ANTONIO LÓPEZ | Tungsteno
In the mid-19th century, the growth of the railway, driven by the Industrial Revolution, clashed with the costly and very slow process of converting cast iron into steel. Then the Englishman Henry Bessemer discovered how to manufacture high quality and durable steel on a large scale, which would not only serve to expand the rail networks, but also mark the beginning of the era of modern cities.
Henry Bessemer (1813-1898) was the son of an illustrious engineer, Anthony Bessemer, a Londoner living in Paris who became a member of the French Academy of Sciences. The French Revolution forced him to return to his native England, where his son Henry was born, a man who would go down in history as the "wizard" of the steel industry and one of the architects of the new world that was to be built with girders and railway tracks made from this material.
In reality, Bessemer's magic was pure chemistry, but in the mid-19th century the scientific keys to metallurgy, a technique that was still based on craftsmanship, were unknown. Blacksmiths had been accumulating traditional knowledge about how to work metals and their alloys for centuries, with the difficulty that the two main qualities of metals—strength and malleability—were usually mutually exclusive. These craftsmen sought a balance between shaping the metals with ease and creating pieces that were as durable as possible. And their challenging craft had the added difficulty that native metals, as found in nature, often also contain impurities and traces of other substances, something that could be confirmed thanks to the experiments of Bessemer and other engineers.
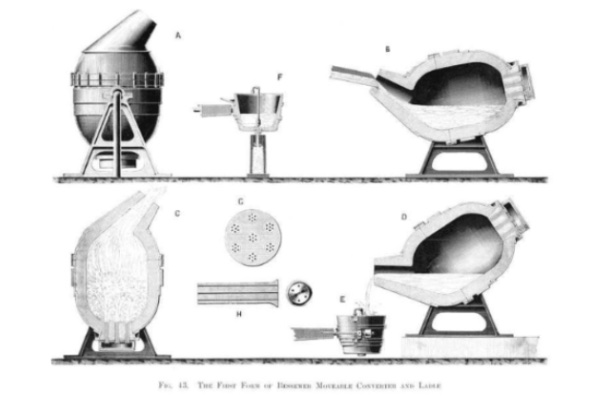
The method devised by Bessemer removed the impurities from the iron thanks to a process of oxidation of the air, obtaining a more resistant steel much more quickly. Credit: Wikimedia Commons.
A military stimulus to save production time
At the height of the Crimean War (1853-1856), England sought to produce stronger steel in large quantities, as some cast-iron cannon barrels could not withstand the force generated by new, more powerful shells. Steel is not a metal, but a mixture of cementite and ferrite which, if it contains an excessive percentage of carbon, will fail.
It took Bessemer several attempts to figure out the chemistry and find the right formula to produce durable steel, including a disastrous out-of-control volcanic-like eruption in 1855 that burnt part of the building’s roof; nevertheless, that dangerous experiment confirmed his hypothesis that the impurities in the iron could be removed by a process of oxidation with air. Bessemer introduced a blast of air into a bath of molten iron, which in addition to raising the temperature of the melted iron, produced a combustion that burned the impurities and carbon in the iron (which could then be artificially introduced in the exact proportion desired). With his new method of oxidation, he could convert 25 tons of iron into steel in 25 minutes.
A year after he patented his method (1855), Henry Bessemer and Company went into business and presented his invention to the British Association for the Advancement of Science. However, the Bessemer converter (the clay-coated furnace he had designed to carry out his decarbonisation process) exposed some flaws in the process: phosphorus and sulphur were not eliminated. It wasn't until 1877 that British metallurgist Sidney Gilchrist Thomas developed a lining for the converter that removed phosphorus and enabled the use of the European continent's phosphoric minerals. Over time, the Bessemer method was refined thanks to the contributions of other engineers, such as Robert Mushet's discovery that adding manganese made it easier to forge steel.

The Bessemer process dramatically lowered the cost of producing steel, which contributed to the emergence of the railroad. Credit: California State Railroad Museum Library.
Bessemer and the steel belt around the Earth
With the drop in demand for steel after the war, Bessemer focused on a more promising long-term market: the railroads. His steel train tracks could be used for an average of 18 years, as opposed to four years for iron ones, so the railroad spread quickly; while in 1840 there were 5,353 kilometres of railroads in the United States, by 1860 there were 49,246 km, which put in a straight line would circle the globe (the circumference of the Earth at the equator is 40,075 km). Bessemer consolidated his success as an entrepreneur via rails, capturing 80% of the market share of rail steel between 1880 and 1895. By then, his steel tracks could go around the equator 10 times.
In 1898, the magazine Scientific American published an article that analysed precisely the important economic effects of the increase in the supply of cheap steel. In the end, the Bessemer process decreased its cost of production from 40 to six or seven pounds sterling per ton. Steel and the elevator led to the construction of skyscrapers and department stores, which would become the centre of commercial activity and even transform social customs—beyond what was prescribed by tradition and religion. As The New York Times noted in 1890, the "epidemic of giving and receiving presents" at Christmas had begun.
· — —
Tungsteno is a journalism laboratory to scan the essence of innovation. Devised by Materia Publicaciones Científicas for Sacyr’s blog.
